Comprehensive Strengths
The EPNextS Group was founded in 1991 as a pioneer in the domestic contract research organization (CRO) industry. Since then, we have expanded our diverse services, including Site Management Organization (SMO) and Contract Sales Organization (CSO), with a primary focus on CRO. We provide end-to-end support solutions from the development of pharmaceuticals, medical devices, and regenerative medicine products to post-marketing surveillance.
Building on the foundation of our clinical trial and post-marketing businesses, which boast a top-tier scale and proven track record in Japan, we are actively engaging in data business and new ventures, constantly striving for evolution.
The driving force behind this is the "OneEPS" culture rooted throughout the group. Close cooperation across departments and companies allows us to manage information and quality seamlessly. Guided by our three values of "For our Clients, For our Business, For our People," we leverage our advanced expertise and comprehensive strengths to deliver optimal proposals and swift responses.
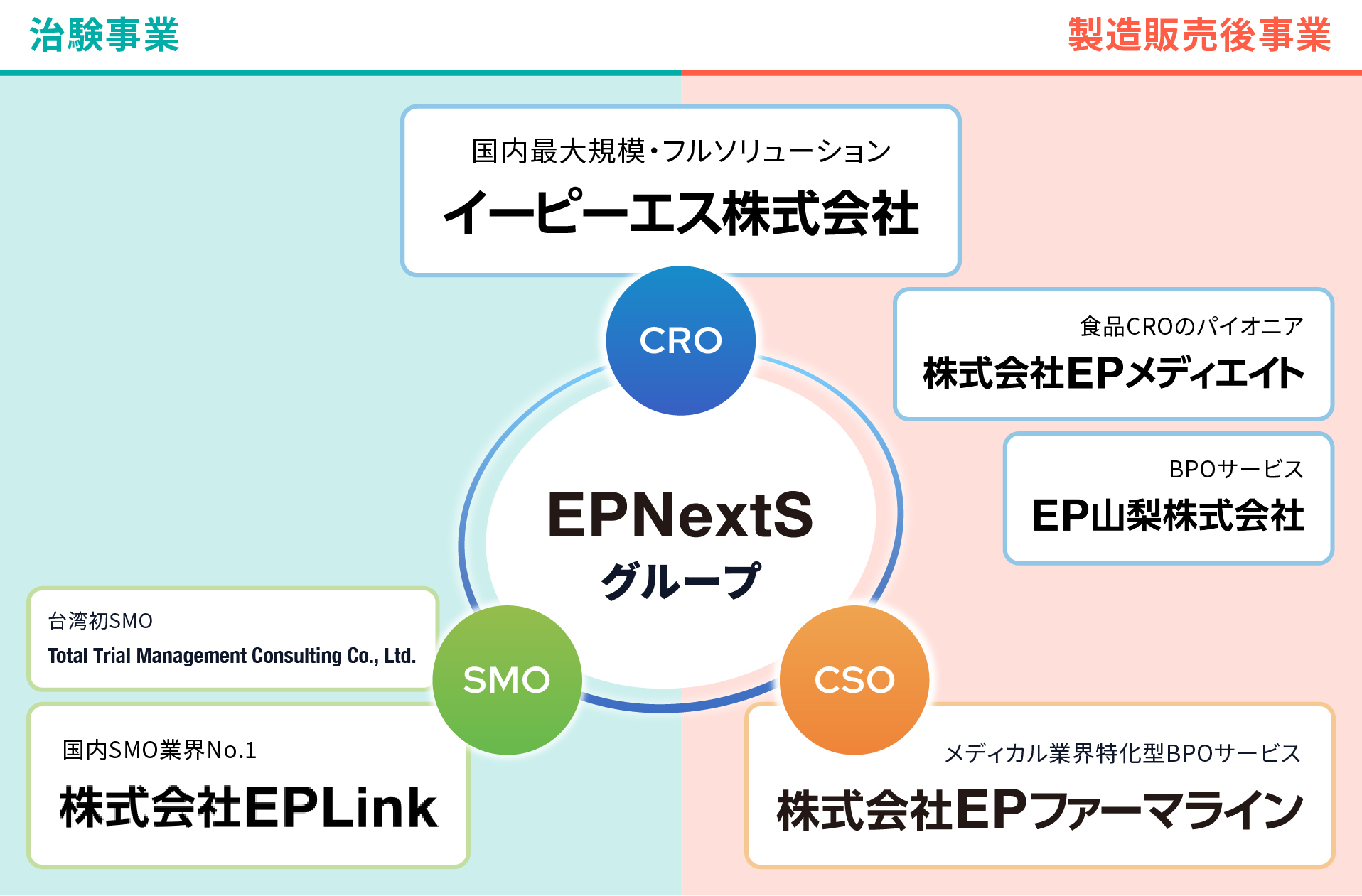
We provide full-service solutions that promote the entire lifecycle from the development of pharmaceuticals, medical devices, and regenerative medicine products to post-marketing surveillance, including clinical research.
Comprehensive
Strengths
01
The EPNextS Group’s Advantage
Advantage01
Japan's Largest-Scale Clinical Trials Support System
-
EPNextS Group
Employees
Approx.
7,000 -
EPNextS Group
Qualified Professionals(Pharmacists, Clinical Laboratory Technicians, Nurses)
Approx.
2,500 -
EPS Corp.
CRA(Clinical Research Associate)
Over
1,100 -
EPS Corp.
Data Manager(Largest team among domestic CROs)
Over
500 -
EP-Link
CRC(Clinical Research Coordinator)
Over
1,300 -
EP-Link
Affiliated Medical Institutions Nationwide
7,200Sites
Advantage02
High Expertise and Site Coverage in Oncology
-
EPS Corp.
CRA Specializing in Oncology Trials
Approx.
700 -
EPS Corp.
Monitoring Operations
Oncology TrialsApprox.
70%
Proportion of Active Studies -
EPS Corp.
Japan CRO Association
Oncology TrialsApprox.
36%
Source: Japan CRO Association Annual Report 2023 -
EP-Link
CRC Specializing in Oncology Trials
Approx.
600 -
EP-Link
National Cancer Centers
Partnering with 18Out of
18National
Cancer Centers -
EP-Link
MHLW-Designated Cancer Care Hospitals
Out of 456 SitesPartnering
with Approx.80%
Advantage03
High-Quality Services Supported by Qualified Medical Professionals
-
High-Quality Services by Qualified Medical Professionals
Medical Contact CenterOver
300Companies
-
24
/
7Comprehensive Support System
-
Qualified Professionals(Pharmacists, Nurses, Medical Representatives (MRs), and Registered Dietitians/Dietitians)
Over
1,025
-
Development Support of Foods with Health Claims
Human Efficacy and Safety Assessment
Over
1,600Studies
Basic Research and Animal Testing
Over
800Studies
-
Publications Related to Foods with Health Claims Development
Over
1,600
-
Clinical Trials Support
Over
700Trials
-
Global Trials
90%
Comprehensive
Strengths
02
Training System for Professional Development
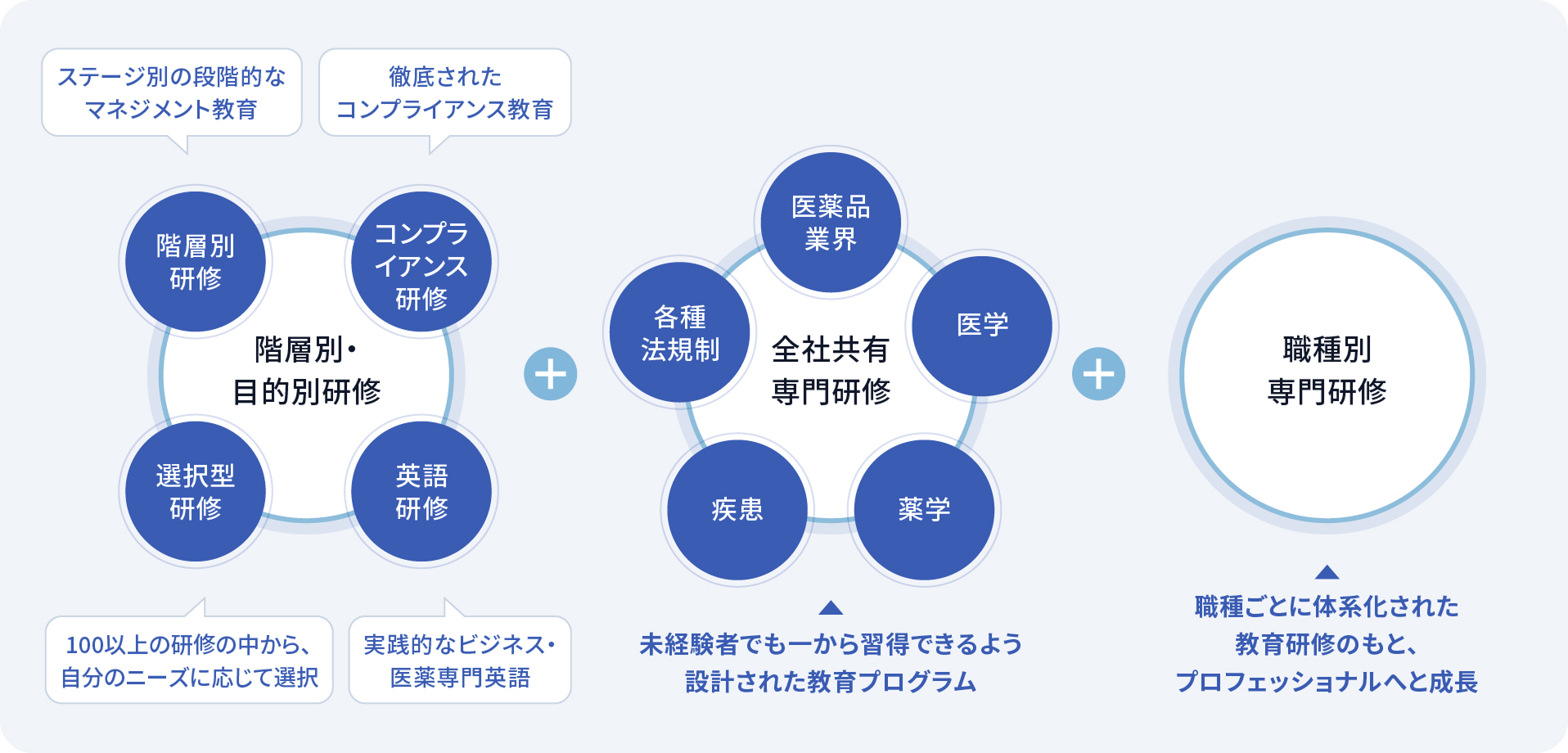
The EPNextS Group has established a comprehensive training system that combines level- and role-specific training, e-learning, and group training to ensure steady growth while enhancing expertise in pharmaceutical development. We have a rooted culture of practical learning, extending beyond lectures, where the entire assigned mentors and project team provide support. This creates an environment where even those with no prior experience can gain deep knowledge through on-the-job experience. With a wide range of programs covering language skills, regulations, and management, employees can continuously refine their expertise and achieve career-based growth not only upon joining the company but also throughout their careers.
Comprehensive
Strengths
03
EPNextS Group: Challenging Ourselves to Create New Value
The EPNextS Group is continuously pursuing the creation of "new business models" for the next stage. This persistent drive is supported by the proven track record established by each Group company and by "Comprehensive Strengths of the EPNextS Group," which are cultivated by the expertise and experience of every single employee. To achieve our mission, "We will contribute to the advancement of the health industry by providing high-value-added solutions to our clients," we are enhancing our information gathering and strategy development to adapt to changing environments. Moving beyond conventional pharmaceutical development support, we are committed to creating social value centered on health and will provide new business solutions across the healthcare field as a company that supports the future well-being of people around the world.
Awards & Recognition
2025
-
January
Recognized as one of the top 10 companies providing exemplary services and solutions in the Medical Affairs field.
2024
-
SeptemberEP-PharmaLine2024 Jury's Special Award, Contact Center Award
Received this award for our successful initiatives to prevent early turnover and accelerate skill acquisition. These efforts, which include the “Human Resources Support Plan” for first-year Medical Communicators and measures implemented during the recruitment process, were highly praised as a model strategy widely referenced across the industry.
-
March
Received the Award of Excellence at the “5th YAMANASHI Working Style Award,” hosted by the Department of Industry and Labor, Yamanashi Prefectural Government.
-
January
Selected as one of the top 10 companies in the Asia-Pacific region. This recognition was based on our proven track record as one of the few CROs in Japan capable of comprehensively supporting the entire lifecycle of regenerative medicine products. We were specifically recognized as a frontline company providing regenerative medicine solutions and significantly impacting the market.
2023
-
SeptemberEP-PharmaLine2023 Jury's Special Award, Contact Center Award
Received this award for the “URGENT Communicator®” service, which enables the immediate establishment of a contact center to rapidly respond to urgent cases, such as pharmaceutical shipment adjustments and product recalls. This initiative received high praise.
-
AugustEP-PharmaLineTop Pharma Consulting in Japan 2023
Recognized for our comprehensive support for medical management in Japan and our strength in adapting our business to the unique characteristics of the Japanese healthcare industry.
-
January
Our development group received the Award for Excellence for the theme: “Clinical Trial DX! Achieving Efficiency in Pharmaceutical Clinical Trial Business Processes with Game-Changing Convenience and Ultra-High Security.”
The system jointly developed with BitBrain Corporation, “SPG-Remote Medical for SYNOV-R,” was recognized for solving critical challenges in the clinical trial process. This development is expected to contribute to the stable supply of effective new drugs to be developed in Japan in the future.
2022
-
SeptemberEP-PharmaLine2022 Best Operation Award, Contact Center Award
The “ES Navigation®” service, which combines the “expertise” of EP-PharmaLine with the “mobility” of Marketing Specialist (MS), received high praise as a new scheme for information provision activities that addresses recent changes in the healthcare industry environment.
2021
-
SeptemberEP-PharmaLine2021 Jury's Special Award, Contact Center Award
Our initiative, named “Creating an environment where specialized personnel can thrive and ensuring employment stability through organizational support,” was recognized as a reference case for the treatment and support of knowledge workers essential to highly advanced contact centers.
2019
-
September
EP-PharmaLine developed the “Medical Communicator Certification” to standardize knowledge and skills in contact center operations. This initiative was highly evaluated for successfully improving response quality and fostering human resources development.
















